SHP-1GST - full length
Src homology 2 domain Phosphatase-1
human, recombinant, E. coli
| Catálogo Nº | Apresentação | Preço (R$) | Comprar / Observação |
|---|---|---|---|
| PR-947 | 20 μg | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped on dry ice
Condições de armazenamento: store at -80 °C
avoid freeze/thaw cycles
Validade: 12 months
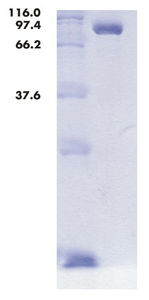
Peso molecular: 92 kDa
Número de acesso: NP_002822.2
Número de acesso: NP_002822.2
Pureza: > 90 % (SDS-PAGE)
Forma: liquid (Supplied in 50 mM Tris-HCl pH 8.0, 100 mM NaCl and 1 mM DTT)
Atividade: Basal activity (unstimulated): 130 pmol Pi/min/μg; Phosphatase activity was determined with pNPP as substrate at pH 7.5 and 30°C.(see PR-944) The enzyme was used in final concentrations of 15 and 30 nM.
Descrição:
The N-terminal GST-tagged fusion protein was expressed in E. coli and purified by affinity chromatography with GSH-beads. The GST-tag influences to some degree the stimulation by ligands of the N-terminal SH2-domain can dimerize and even be phosphorylated. The enzyme should only be used in diluted solutions or by adding 10% glycerol. SHP-1 (Src homology-2 containing protein tyrosine phosphatase-1) is a non-receptor protein tyrosine phosphatase with two phosphotyrosine binding domains. N- and C-terminal tandem SH2 domains lie N-terminal to the catalytic domain (PTP). In the unstimulated state interaction of the N-terminal SH2 domain with the catalytic domain leads to self inhibition. Natural ligand sequences from cytosolic parts of receptors, signal and scaffold proteins or synthetic phosphotyrosine peptides stimulate the phosphatase activity. Thus, SHP-1 acts as negative regulator in the signaling of various receptors, including erythropoietin receptor, IL3-receptor, CSF-1 receptor, B-cell receptor and c-Ros. SHP-1 prefers as substrate such proteins which are phosphorylated from the SRC-kinase. SHP-1 can act as tumor suppressor or can inhibit the processing of some immune cells.
Referências selecionadas:
Zhang et al. (2000) Roles of SHP-1 tyrosine phosphoatase in the negative regulation of Cell signallimg. Immunology 12:361. Keilhack et al. (2001) Negative regulation of Ros receptor tyrosine kinase signaling. An epithelial function of the SH2 domain protein tyrosine phosphatase SHP-1. J. Cell Biol. 152:325. Li et al. (2000) Form, function, and regulation of protein tyrosine phosphatases and their involvement in human diseases. Semin. Immunol. 12:75. Wu et al. (2003) SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. J. Cell Biochem. 90:1026. Kamata et al. (2003) Src-homology 2 domaincontaining tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J. Clin. Invest. 111:109. Yang et al. (1998) Crystal structure of the catalytic domain of protein-tyrosine phosphatase SHP-1. J. Biol. Chem. 273:28199. Yang et al. (2000) Structural basis for substrate specificity of protein-tyrosine phosphatase SHP-1.
