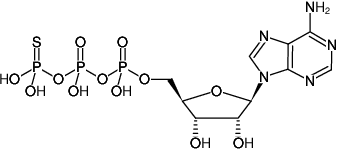
ATPγS
Adenosine-5'-(γ-thio)-triphosphate, Tetralithium salt
Adenosine-5'-(3-thio)-triphosphate, Adenosine-5'-(3-thiotriphosphate)
Ado-5'-PPP[S]
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| NU-406-5 | 5 mg | Sob demanda | Adicionar ao Carrinho |
| NU-406-25 | 25 mg | Sob demanda | Adicionar ao Carrinho |
| NU-406-50 | 50 mg | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped on dry ice
Condições de armazenamento: store at -20 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 6 months after date of delivery
Fórmula molecular: C10H16N5O12P3S (free acid)
Peso molecular: 523.24 g/mol (free acid)
CAS#: 93839-89-5
Pureza: ≥ 90 % (HPLC), contains < 10 % ADP
Forma: solid
Propriedades espectroscópicas: λmax 259 nm, ε 15.4 L mmol-1 cm-1 (Tris-HCl pH 7.5)
Formulários:
Modulation of intracellular signalling[1,2]
Signalling of purinergic receptors[3]
Regulation of cation channels[4]
Modulation of cytokine secretion[5]
Substrate for kinases[6]
5' end labeling with T4 Polynucleotide Kinase (T4 PNK) of DNA[9] and RNA[9,10]
Agonistic ligand, mainly for nucleoside receptor A1
Nucleosidephosphates stabilized against hydrolytic degradation can directly bind to nucleoside receptors.
Specific Ligands:
Purinoreceptors[7]
Proteasomes[8]
Agonist for P2Y2[11] and 12 receptors[1,15] and for P2X(2) purinoreceptor[3,12,13,14]
Please note: For reasons of stability, please make sure that the pH value of a solution of this product never drops below 7.0. This can be achieved by dissolving the nucleotide in a buffer of your choice (50 - 100 mM, pH 7 - 10). Dissolve and adjust concentration photometrically.
Referências selecionadas:
[1] Buzzi et al. (2009) Extracellular ATP activates MAP kinase cascades through a P2Y purinergic receptor in the human intestinal Caco-2 cell line. Biochim. Biophys. Acta 1790:1651.
[2] Wu et al. (2008) Extracellular ATP-induced NO production and ist dependence on membrane Ca2+ flux in Salvia miltiorrhiza hairy roots. J. Experim. Botany 59:4007.
[3] Hansen et al. (2008) Purinergic receptors and calcium signalling in human pancreatic duct cell lines. Cell. Phys. Biochem. 22:157.
[4] Ashrafpour et al. (2008) ATP regulation of a large conductance voltage-gated cation channel in rough endoplasmatic reticulum of rat hepatocytes. Arch. Biochem. Biophys. 471:50.
[5] Nalos et al. (2008) Host tissue damage signal ATP impairs IL-12 and IFNgamma secretion in LPS stimulated whole human blood. Intensive care medicine 34:1891.
[6] Carlson et al. (2010) Use of a semisynthetic epitope to probe histidine kinase activity and regulation. Anal. Biochem. 397:139.
[7] Choi et al. (2009) Modulation of firing activity by ATP in dopamine neurons of the substantia nigra pars compacta. Neuroscience 160:587.
[8] Wang et al. (2009) Structural insights on the mycobacterium tuberculosis protosomal ATPase Mpa. Structure 17:1377.
[9] Grant et al. (2007) A facile method for attaching nitroxide spin labels at the 5' terminus of nucleic acids. Nucleic Acids Research 35 (10):e77.
[10] Akabayov et al. (2002) Immobilization of RNA: Application to Single Molecule Spectroscopy. Molecular, Cellular and Tissue Engineering, 2002. Proceedings of the IEEE-EMBS Special Topic Conference:71.
[11] Gorodeski (2002) Regulation of transcervical permeability by two distinct P2 purinergic receptor mechanisms. Am. J. Physiol. Cell Physiol. 282 (1):C75.
[12] Bo et al. (1994) Comparative studies on affinities of ATP derivatives for P2X-purinoreceptors in rat urinary bladder. Br. J. Pharmacol. 112 (4):1151.
[13]He et al. (2002) Purinergic P2X (2) receptor desensitization depends on coupling between ectodomain and C-terminal domain. Molec. Pharmac. 62 (5):1187.
[14]Evans et al. (1995) Pharmacological characterizationof heterogously expressed ATP-gated cation channels (P2X purinoreceptors). Mol. Pharmacol. 48:178.
[15] Isfort et al. (2011) Real-time imaging reveals that P2Y2 and P2Y12 receptor agonists are not chemoattractants and macrophage chemotaxis to complement C5a is phosphatidylinositol 3-kinase (PI3K)- and p38 mitogen-activated protein kinase (MAPK)-independent. J. Biol. Chem. 288 (52):44776.
Schmohl et al. (2012) Functional analysis of Rho GTPase activation and inhibition in a bead-based miniaturized format. Methods Mol. Biol. 827:271.
Fischmann et al. (2009) Crystal Structures of MEK1 Binary and Ternary Complexes with Nucleotides and Inhibitors. Nucleic Acids Research 48 (12):2661.
Del Toro Duany et al. (2008) The reverse gyrase helicase-like domain is a nucleotide-dependent switch that is attenuated by the topoisomerase domain. Nucleic Acids Research 36 (18):5882.
Wong et al. (2006) Endogenous activation of adenosine A1 receptors, but not P2X receptors, during high frequency synaptic transmission at the calyx of Held. J. Neurophysiology 95 (6):3336.
Chang et al. (2005) Nitric Oxide-dependent Allosteric Inhibitory Role of a Second Nucleotide Binding Site in Soluble Guanylyl Cyclase. J. Biol. Chem. 280 (12):11513.
Matamoros et al. (2005) Suppression of Multidrug-resistant HIV-1 Reverse Transcriptase Primer Unblocking Activity by α-Phosphate-modified Thymidine Analogues. J. Mol. Biol. 349 (3):451.
Masino et al. (2002) Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine A1 receptors. J. Pharmacol. and Experiment. Therapeutics 303 (1):356.
