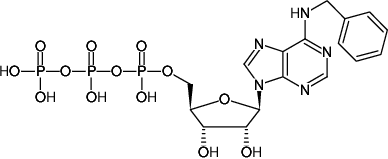
N6-Benzyl-ATP
N6-Benzyl-adenosine-5'-triphosphate, Sodium salt
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| NU-1196S | 300 μl (10 mM) | Sob demanda | Adicionar ao Carrinho |
| NU-1196L | 5 x 300 μl (10 mM) | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped on gel packs
Condições de armazenamento: store at -20 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C17H22N5O13P3 (free acid)
Peso molecular: 597.30 g/mol (free acid)
CAS#: 40922-97-2
Pureza: ≥ 95 % (HPLC)
Forma: solution in water
Concentração: 10 mM - 11 mM
pH: 7.5 ±0.5
Propriedades espectroscópicas: λmax 269 nm, ε 20.5 L mmol-1 cm-1 (Tris-HCl pH 7.5)
Formulários:
Agonistic ligand, mainly for nucleoside receptor A1, with less affinity to A2A and A3
Nucleoside-triphosphates can be converted by different membrane-bound phosphatases into nucleosides acting as nucleoside receptor ligands. In some cases nucleoside phosphates act also directly on nucleoside receptors.
Referências selecionadas:
Sirci et al. (2012) Ligand-, structure- and pharmacophore-based molecular fingerprints: a case study on adenosine A1, A2A, A2B, and A3 receptor antagonists. J. Comput. Aided Mol. Des. 26:1247.
Volonte et al. (2009) Membrane components and purinergic signalling: the purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. FEBS J. 276:318.
Yegutkin (2008) Nucleotide and nucleoside converting enzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783:673.
Joshi et al. (2005) Purine derivatives as ligands for A3 adenosine receptors. Current Topics in Medicinal Chemistry 5:1275.
Zhou et al. (2005) High affinity ATP/ADP analogues as new tools for studying CFTR gating. J. Physiol. 569 (2):447.
Hess (2001) Recent advantages in adenosine receptor antagonist research. Expert Opin. Ther. Patents 11 (10):1533.
Jacobson (2001) Probing adenosine and P2 receptors: design of novel purines and nonpurines as selective ligands. Drug Development Res. 52:178.
Jacobson et al. (2001) Ribose modified nucleosides and nucleotides as ligands for purine receptors. Nucleosides, Nucleotides & Nucleic Acids 20 (4):333.
Gillespie et al. (1999) Engineering of the myosin-ibeta nucleotide-binding pocket to create selective sensitivity to N (6)-modified ADP analogs. J. Biol. Chem. 274 (44):31373.
Van Galen et al. (1994) A binding site model and structure-activity relationships for rat A3 adenosine receptor. Molecular Pharmacology 45:1101.
