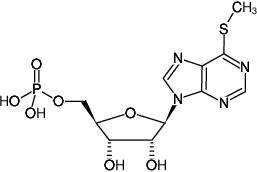
6-Methylthio-IMP
6-Methylthioinosine-5'-monophosphate, Triethylammonium salt
6-Methylmercapto-9-(β-D-ribofuranosyl)purine-5'-monophosphate
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| NU-1131S | 150 μl (10 mM) | Sob demanda | Adicionar ao Carrinho |
| NU-1131L | 5 x 150 μl (10 mM) | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped on gel packs
Condições de armazenamento: store at -20 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C11H15N4O7PS (free acid)
Peso molecular: 378.29 g/mol (free acid)
CAS#: 7021-52-5
Pureza: ≥ 95 % (HPLC)
Forma: solution in water
Concentração: 10 mM - 11 mM
pH: 7.5 ±0.5
Propriedades espectroscópicas: λmax 224/292 nm, ε 11.5/19.0 L mmol-1 cm-1 (Tris-HCl pH 7.5)
Formulários:
Catabolism by purine-5'-nucleotidase[1]
Transport by multidrug resistance proteins MRP4 and MRP5[2]
Strong inhibitor of purine synthesis de novo[3]
Referências selecionadas:
[1] Brouwer et al. (2005) Role of 5'-nucleotidase in thiopurine metabolism:Enzyme kinetic profile and association with thio-GMP levels in patients with acute lymphoblastic leukemia during 6-mercaptopurine treatment. Clinica Chemica Acta 361:95.
[2] Wielinga et al. (2002) Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Molecular Pharmacology 62:1321.
[3] Stet et al. (1994) Decrease of S-adenosylmethionine synthesis by 6-mercaptopurine ribonucleoside in Molt F4 human malignant lymphoblasts. Biochemical J. 304:163.
Lavi et al. (1985) A rapid and sensitive high-performance liquid chromatographic assay for 6-mercaptopurine metabolites in red blood cells. Anal. Biochem. 144 (2):514.
