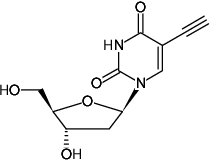
5-Ethynyl-2'-deoxyuridine (5-EdU)
5-Ethynyl-2'-deoxyuridine
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| CLK-N001-25 | 25 mg | Sob demanda | Adicionar ao Carrinho |
| CLK-N001-100 | 100 mg | Sob demanda | Adicionar ao Carrinho |
| CLK-N001-500 | 500 mg | Sob demanda | Adicionar ao Carrinho |
| CLK-N001-5000 | 5 g | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped at ambient temperature
Condições de armazenamento: store at -20 °C
store dry and under inert gas
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C11H12N2O5
Peso molecular: 252.23 g/mol
CAS#: 61135-33-9
Pureza: ≥ 98 % (HPLC)
Forma: solid
Solubilidade: DMSO, up to 200 mM (at room temperature) in 1 x PBS or water by heating the obtained suspension for 1 minute by 70 °C
Propriedades espectroscópicas: λmax 288 nm, ε 12.0 L mmol-1 cm-1 (Tris-HCl pH 7.5)
Formulários:
DNA synthesis monitoring[1-5]
Descrição:
5-EdU (5-Ethynyl-2'-deoxyuridine) can be used as a replacement for BrdU (5-Bromo-2'-deoxyuridine) to measure de novo DNA synthesis during the S-phase of the cell cycle. 5-EdU is cell permeable and incorporates into replicating DNA instead of its natural analog thymidine. The resulting ethynyl-functionalized DNA can subsequently be detected via Cu(I)-catalyzed click chemistry that offers the choice to introduce a Biotin group (via Azides of Biotin) for subsequent purification tasks or a fluorescent group (via Azides of fluorescent dyes) for subsequent microscopic imaging [1-5].
Presolski et al.[6] and Hong et al.[7] provide a general protocol for Cu(I)-catalyzed click chemistry reactions that may be used as a starting point for the set up and optimization of individual assays.
Produtos relacionados:
- 5-Ethynyl-deoxycytidine (5-EdC), #CLK-N003
- Copper (II)-Sulphate (CuSO4), #CLK-MI004
- Tris(3-hydroxypropyltriazolylmethyl)amine (THPTA), #CLK-1010
- Sodium Ascorbate (Na-Ascorbate), #CLK-MI005
Referências selecionadas:
[1] Salic et al. (2008) A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 105 (7):2415.
[2] Li et al. (2010) Fluorogenic click reaction for labeling and detection of DNA in proliferating cells. Biotechniques 49 (1):525.
[3] Chehrehasa et al. (2009) EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J. Neurosci. Methods 177:122.
[4] Limsirichaikul et al. (2009) A rapid non-radioactive technique for measurement of repair synthesis in primary human fibroblasts by incorporation of ethynyl deoxyuridine (EdU). Nucleic Acids Res. 37 (4):e31.
[5] Buck et al. (2008) Detection of S-phase cell cycle progression using 5-ethynyl-2'-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2'-deoxyuridine antibodies. Biotechniques 44 (7):927.
[6] Presolski et al. (2011) Copper-Catalyzed Azide-Alkyne Click Chemistry for Bioconjugation. Current Protocols in Chemical Biology 3:153.
[7] Hong et al. (2011) Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 48:9879.
