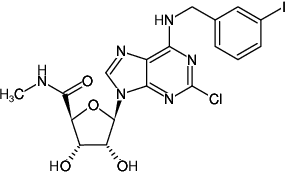
Chloro-IB-MECA
2-Chloro-N6-(3-iodobenzyl)-adenosine-5'-N-methyluronamide
| Catálogo Nº | Apresentação | Preço (R$) | Comprar / Observação |
|---|---|---|---|
| N-1075 | 200 μl (1 mM) | Sob demanda | Adicionar ao Carrinho |
For research use only!
Envio: shipped at ambient temperature
Condições de armazenamento: store at 4 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 24 months after date of delivery
Fórmula molecular: C18H18ClIN6O4
Peso molecular: 544.73 g/mol
CAS#: 163042-96-4
Pureza: ≥ 95 % (HPLC)
Forma: solution in DMSO
Concentração: 1 mM
Formulários:
Binding studies, physiological studies on cell cultures or tissues and in animal experiments.
Compound has high agonistic activity and is highly selective for nucleoside receptor type A3[1,2].
Usage: Dissolve stock solution with aqueous physiological buffer recommended for displacement studies, biochemical signal transduction studies or animal experiments.
To avoid biological effects of DMSO dilute the stock solutions with aqueous buffers to a content of DMSO below 1 %.
Please note: Stable in acidic and neutral buffer solutions for one day
Referências selecionadas:
[1] Brambilla et al. (2000) Activation of A3 adenosine receptor affects cell cycle progression and cell growth. Naunyn-Schmiedeberg's Arch. Pharmacol. 361:225.
[2] Jacobson (2001) Probing adenosine and P2 receptors: design of novel purines and nonpurines as selective ligands. Drug Development Res. 52:178.
Jacobson (2013) Structure based approaches to ligands for G-protein-coupled adenosine and P2Y receptors, from small molecules to nanoconjugates. J. Med. Chem. 56:3749.
Goncalves et al. (2008) Presynaptic adenosine and P2Y receptors. Handbook of Experimental Pharmacology 184:339.
Hasko et al. (2007) Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol.& Therapeutics 113:264.
Ishiwata et al. (2007) PET tracers for mapping adenosine receptors as probes for diagnosis of CNS disorders. Central Nervous System Agents in Medicinal Chemistry 7:57.
Joshi et al. (2005) Purine derivatives as ligands for A3 adenosine receptors. Current Topics in Medicinal Chemistry 5:1275.
Wittendorp et al. (2004) The mouse brain adenosine A1 receptor: functional expression and pharmacology. Europ. J. Pharmacol. 487:73.
Jacobson et al. (2003) Engineering of A3 Adenosine and P2Y Nucleotide Receptors and their ligands. Drug Development Res. 58:330.
Soudijn et al. (2002) Allosteric modulation of G protein-coupled receptors. Current Opinion in Drug Discovery & Development 5 (5):749.
Hess (2001) Recent advantages in adenosine receptor antagonist research. Expert Opin. Ther. Patents 11 (10):1533.
Jacobson et al. (2001) Ribose modified nucleosides and nucleotides as ligands for purine receptors. Nucleosides, Nucleotides & Nucleic Acids 20 (4):333.
Merighi et al. (2001) Pharmacological and biochemical characterization of adenosine receptors in the human malignant melanoma A375 cell line. Brit. J. Pharmacol. 134:1215.
