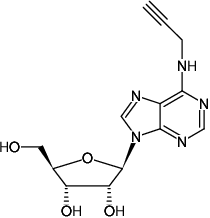
N6-Propargyl-adenosine
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| CLK-N004-1 | 1 mg | Sob demanda | Adicionar ao Carrinho |
| CLK-N004-5 | 5 mg | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped at ambient temperature
Condições de armazenamento: store at -20 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C13H15N5O4
Peso molecular: 305.29 g/mol
Pureza: ≥ 95 % (HPLC)
Forma: solid
Propriedades espectroscópicas: λmax 262 nm, ε 18.0 L mmol-1 cm-1 (Tris-HCl pH 7.5)
Formulários:
mRNA poly(A) tail synthesis monitoring[1]
Descrição:
N6-propargyl-adenosine (N6pA) can be used to measure de novo mRNA poly(A) tail synthesis in proliferating cells. N6pA is cell permeable and incorporates into nascent mRNA transcripts both transcriptionally by RNA polymerase I,II and III and posttranscriptionally by poly(A) polymerase instead of their natural analolg adenosine.
The resulting ethynyl-functionalized RNA can subsequently be detected via Cu(I)-catalyzed click chemistry that offers the choice to introduce a Biotin group (via Azides of Biotin) for subsequent purification tasks or a fluorescent group (via Azides of fluorescent dyes) for subsequent microscopic imaging[1].
Presolski et al.[2] and Hong et al.[3] provide a general protocol for Cu(I)-catalyzed click chemistry reactions that may be used as a starting point for the set up and optimization of individual assays.
Produtos relacionados: 5-Ethynyl-uridine (5-EU), #CLK-N002 Copper (II)-Sulphate (CuSO4), #CLK-MI004 Tris(3-hydroxypropyltriazolylmethyl)amine (THPTA), #CLK-1010 Sodium Ascorbate (Na-Ascorbate), #CLK-MI005
Referências selecionadas:
[1] Grammel et al. (2012) Chemical Reporters for Monitoring RNA Synthesis and Poly (A) Tail Dynamics. Chem Bio Chem 13:1112.
[2] Presolski et al. (2011) Copper-Catalyzed Azide-Alkyne Click Chemistry for Bioconjugation. Current Protocols in Chemical Biology 3:153.
[3] Hong et al. (2011) Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 48:9879.
