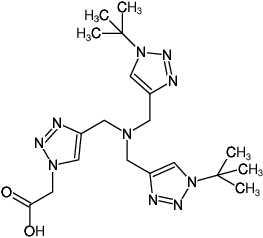
BTTAA
2-(4-((bis((1-(tert-butyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)acetic acid
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| CLK-067-25 | 25 mg | Sob demanda | Adicionar ao Carrinho |
| CLK-067-100 | 100 mg | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped at ambient temperature
Condições de armazenamento: store at -20 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C19H30N10O2
Peso molecular: 430.51 g/mol
Pureza: ≥ 95 % (HPLC)
Forma: solid
Solubilidade: water, DMSO, DMF, MeOH
Descrição:
BTTAA is a water-soluble, very effective ligand for Cu(I)-catalyzed Alkyne-Azide click chemistry reactions (CuAAC). It serves a dual purpose: 1) Accelaration of the CuAAC reaction by maintaining the Cu(I) oxidation state of copper sources and 2) Protection of biomolecules from oxidative damage during the labeling reaction[1,2].
BTTAA is a superior alternative to water-insoluble TBTA.
A stock solution can be prepared in ddH2O and subsequently be stored at -20°C. Avoid freeze-thaw cycles.
Presolski et al.[3] and Hong et al.[4] provide a general protocol for CuAAC reactions that may be used as a starting point for the set up and optimization of individual assays.
Produtos relacionados: Copper (II)-Sulphate (CuSO4), #CLK-MI004 Sodium Ascorbate (Na-Ascorbate), #CLK-MI005 THPTA, #CLK-1010 Picolyl-Azide-PEG4-Biotin, #CLK-1167
Referências selecionadas:
[1] Besanceney-Webler et al. (2011) Increasing the Efficiacy of Bioorthogonal Click Reactions for Bioconjugation: A Comparative Study Angew. Chem. Int. Ed. 50:8051.
[2] Uttamapinant et al. (2012) Fast, Cell-Compatible Click Chemistry with Copper-Chelating Azides for Biomolecular Labeling. Angew. Chem. Int. Ed. 51:5852.
[3] Presolski et al. (2011) Copper-Catalyzed Azide-Alkyne Click Chemistry for Bioconjugation. Current Protocols in Chemical Biology 3:153.
[4] Hong et al. (2011) Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 48:9879.
