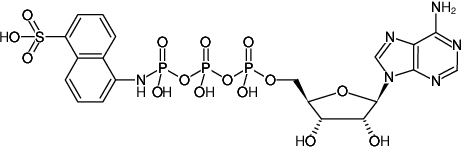
ATP-γ-AmNS
Adenosine-5'-triphosphate-γ-(sulfo-1-naphthyl)amide, Triethylammonium salt
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| NU-1616S | 150 μl (5 mM) | Sob demanda | Adicionar ao Carrinho |
| NU-1616L | 5 x 150 μl (5 mM) | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped on gel packs
Condições de armazenamento: store at -20 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C20H23N6O15P3S (free acid)
Peso molecular: 712.41 g/mol (free acid)
Pureza: ≥ 95 % (HPLC)
Forma: solution in water
Concentração: 5.0 mM - 5.5 mM
pH: 7.5 ±0.5
Propriedades espectroscópicas: λmax 323 nm, ε 4.2 L mmol-1 cm-1 (Tris-HCl pH 7.5), λexc 323 nm, λem 461 nm
Formulários:
Quenching of protein fluorescence[1]
Identification of binding sites in E-coli isocitrate dehydrogenase kinase[1]
Estimation of ADP-binding sites in membrane bound adenine-nucleotide carrier[2]
Substrate for snake venom phosphodiesterase[3]
Intramolecular energy transfer[4]
E-coliIsocitrate dehydrogenase kinase/phosphatase[1]
Referências selecionadas:
[1] Rittinger et al. (1996) Escherichia coli isocitrate dehydrogenase kinase/phosphatase. Overproduction and kinetics of interaction with its substrates by using intrinsic fluorescence and fluorescent nucleotide analogues. Eur. J. Biochem. 237 (1):247.
[2] Block et al. (1984) Substrate-site interactions in the membrane-bound adenine-nucleotide carrier as disclosed by ADP and ATP analogs. Biochim Biophys Acta. 767 (2):369.
[3] Pollack et al. (1982) Fluorescent nucleotide triphosphate substrates for snake venom phosphodiesterase. Anal. Biochem. 127 (1):81.
[4] Yarbrough et al. (1980) Stacking interactions in fluorescent nucleotide analogs containing 1-aminonaphthalene-5-sulfonate at the phosphoryl terminus. J. Biol. Chem. 255 (20):9907.
