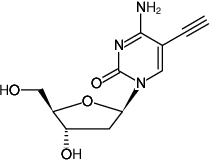
5-Ethynyl-2'-deoxycytidine (5-EdC)
5-Ethynyl-2'-deoxycytidine
| Catálogo Nº | Apresentação | Preço (R$) | Comprar / Observação |
|---|---|---|---|
| CLK-N003-10 | 10 mg | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped at ambient temperature
Condições de armazenamento: store at -20 °C
store dry and under inert gas
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C11H13N3O4
Peso molecular: 251.24 g/mol
Pureza: ≥ 99 % (HPLC)
Forma: solid
Solubilidade: DMSO
Propriedades espectroscópicas: λmax 291 nm, ε 8.5 L mmol-1 cm-1 (Tris-HCl pH 7.5)
Formulários:
DNA synthesis monitoring[1]
Descrição:
Ethynyl-labeled deoxycytidine (5-EdC) can be used as a replacement for BrdU (5-Bromo-2’-deoxyuridine) to measure de novo DNA synthesis during the S-phase of the cell cycle. 5-EdC is cell-permeabel and incorporates into replicating DNA instead of its natural analog thymidine.
The resulting ethynyl-functionalized DNA can subsequently be detected via Cu(I)-catalyzed click chemistry that offers the choice to
introduce a Biotin group (Azides of Biotin) for subsequent purification
tasks or a fluorescent group (Azides of fluorescent dyes) for subsequent
microscopic imaging [1].
Presolski et al.[2] and Hong et al.[3] provide a general protocol for Cu(I)-catalyzed click chemistry reactions that may be used as a starting point for the set up and optimization of individual assays.
Produtos relacionados: 5-Ethynyl-2'-deoxy-uridine (5-EdU), #CLK-N001 Copper (II)-Sulphate (CuSO4), #CLK-MI004 Tris(3-hydroxypropyltriazolylmethyl)amine (THPTA), #CLK-1010 Sodium Ascorbate (Na-Ascorbate), #CLK-MI005
Referências selecionadas:
[1] Guan et al. (2011) Intracellular detection of cytosine incorporation in genomic DNA by using 5-ethynyl-2'-deoxycytidine. ChemBioChem 2 (14):2184.
[2] Presolski et al. (2011) Copper-Catalyzed Azide-Alkyne Click Chemistry for Bioconjugation. Current Protocols in Chemical Biology 3:153.
[3] Hong et al. (2011) Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation. Angew. Chem. Int. Ed. 48:9879.
