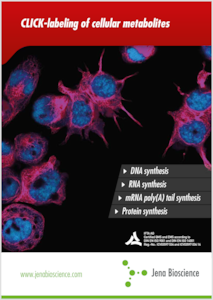
Click Chemistry
Click Chemistry[1] pode ser descrita como pares de grupos funcionais que reagem entre si (“click”) rapidamente e seletivamente em condições aquosas brandas. Click Chemistry tornou-se um procedimento conveniente, versátil e reprodutivo de acoplamento de duas moléculas A e B[1-5] agora amplamente utilizado em ciências da vida[6-8], descobrimento de drogas[9] e ciências materiais[10].
Produtos
Downloads
Selected References
Introdução para o conceito de Click Chemistry
[1] Kolb et al. (2001) Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40 (11):2004.
[2] Sletten et al. (2009) Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed.48:6998.
[3] Jewett et al.(2010) Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 39 (4):1272.
[4] Best et al. (2009) Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Biochemistry.48:6571.
[5] Lallana et al. (2011) Reliable and Efficient Procedures for the Conjugation of Biomolecules through Huisgen Azide–Alkyne Cycloadditions. Angew. Chem. Int. Ed. 50:8794.
Resumo das aplicações de Click Chemistry
[6] Grammel et al. (2013) Chemical Reporters for biological discovery. Nature Chemical Biology 9:475.
[7] Xie et al. (2013) Cell-selective metabolic labeling of biomolecules with bioorthogonal functionalities. Current Opinion in Chemical Biology 17:747.
[8] Su et al. (2013) Target identification of biologically active small molecules via in situ methods. Current Opinion in Chemical Biology 17:768.
[9] Zeng et al. (2013) The Growing Impact of Bioorthogonal Click Chemistry on the Development of Radiopharmaceuticals. J Nucl Med 54:829.
[10] Evans et al. (2007) The Rise of Azide–Alkyne 1,3-Dipolar 'Click' Cycloaddition and its Application to Polymer Science and Surface Modification. Australian Journal of Chemistry 60 (6):384.